Spectacular Tips About How To Find Out How Many Electrons Are In An Element

In this case, since the carbon has only three bonds and a negative charge, it must also have a.
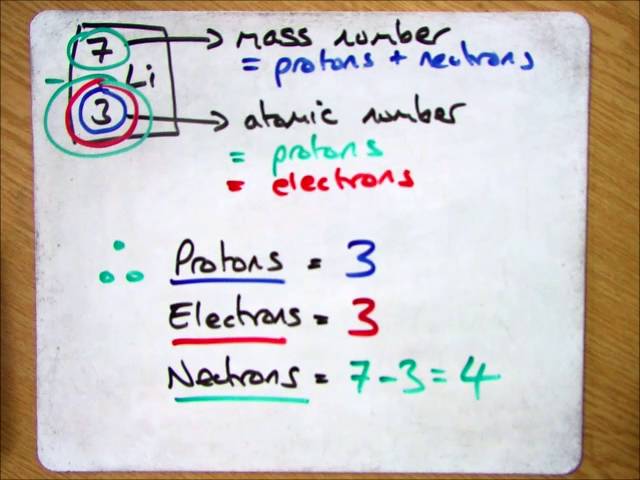
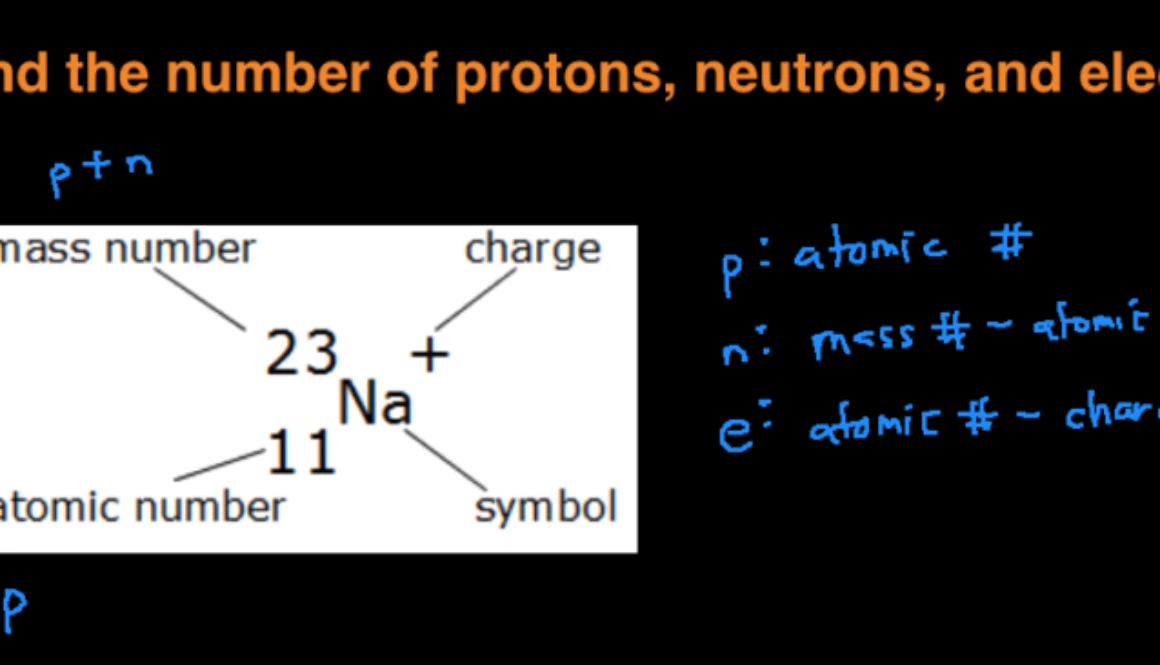
How to find out how many electrons are in an element. How do you find the number of protons electrons and neutrons in an element? Elements in group 13 have three valence electrons;. To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
A negatively charged carbon atom should immediately tell you about a lone pair of electrons. We know that an equal number of protons of atomic number are located in the nucleus of the element and electrons equal to protons are in orbit outside the nucleus. If the ion is negatively charged, the number of electrons is found by adding the charge number to the proton number.
Elements in group 2 have two valence electrons; Looking at the periodic table, atoms have a regularly occurring number of valence electrons based on their group number. In this case, there are fewer protons than electrons.
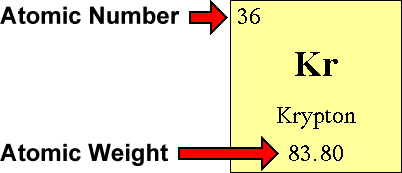
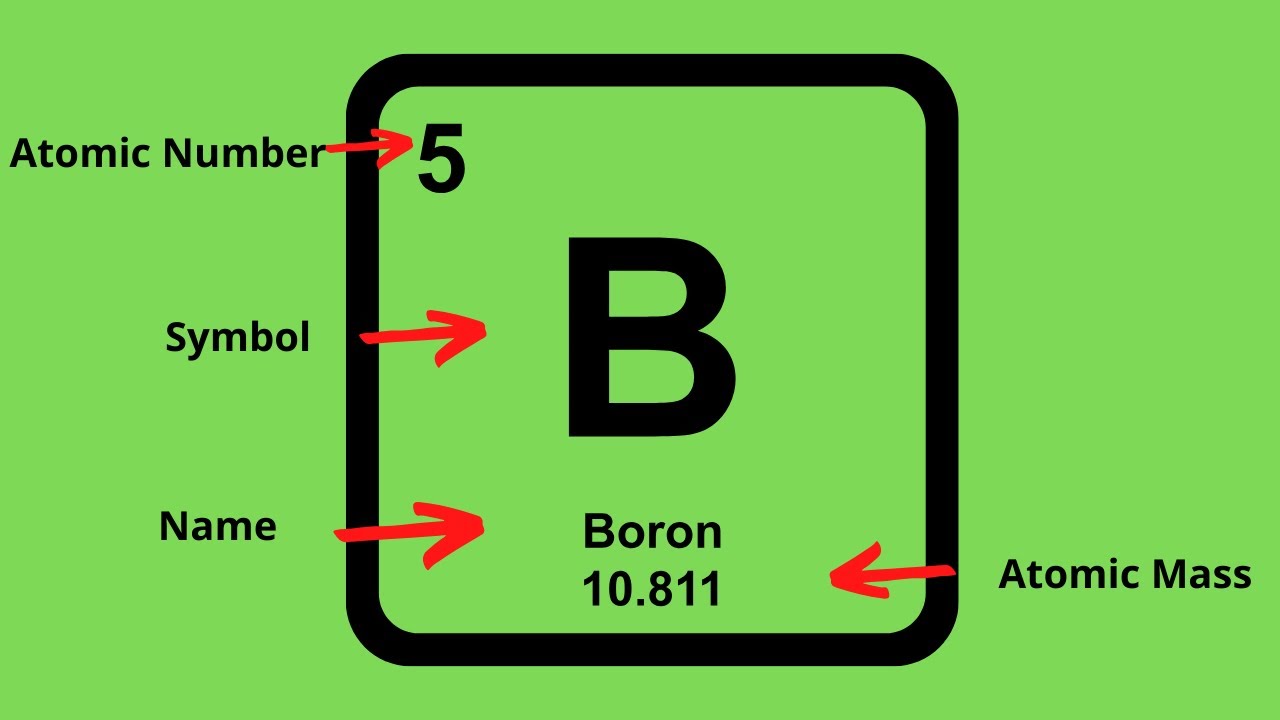
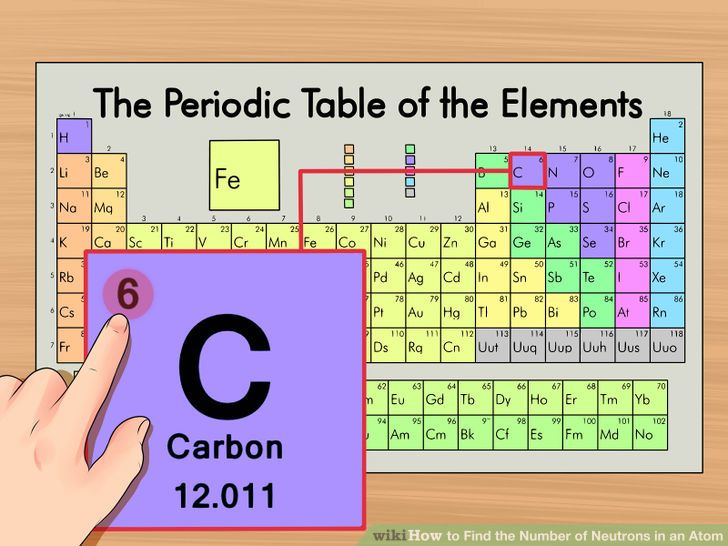
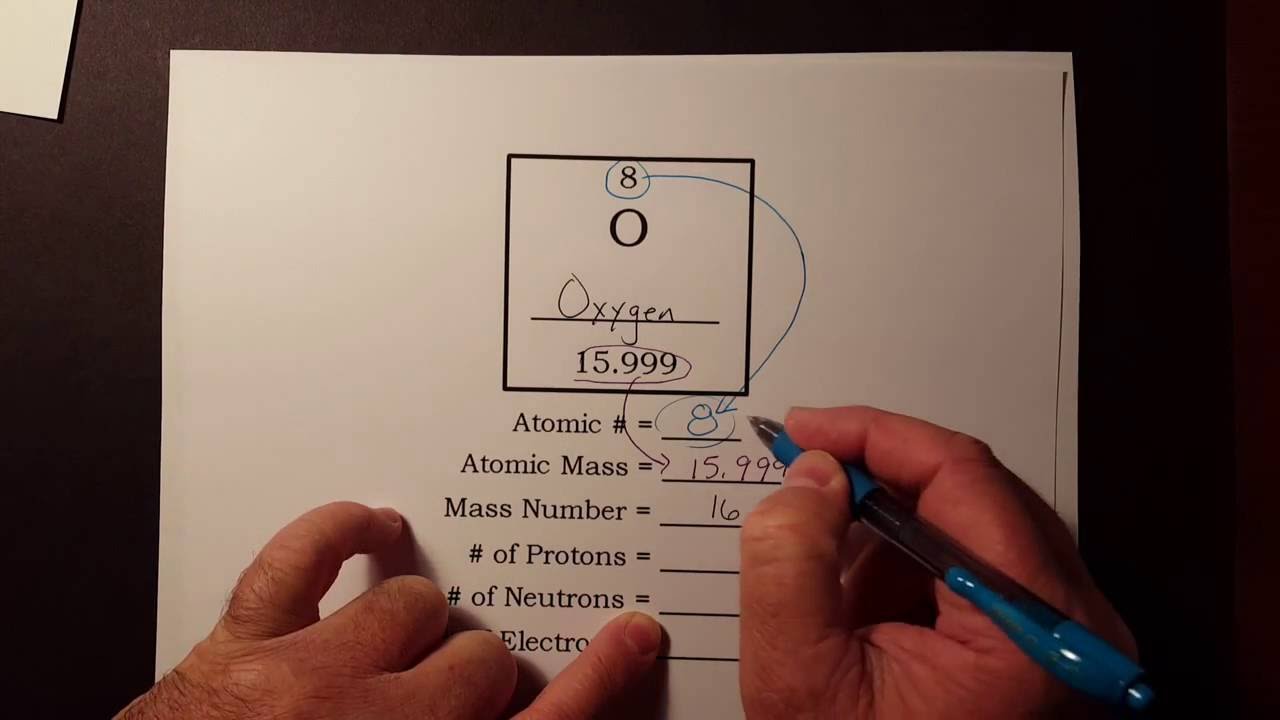
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass. The number of protons in the nucleus of the atom is equal to the atomic number (z). To determine how many total electrons there are, add the amount of charge to the atomic number.
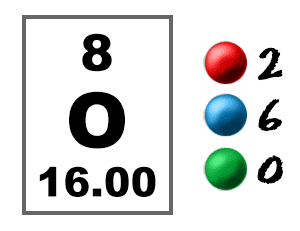
Since the atom is neutral, the number of electrons is equal to the proton. We know that an equal number of protons of atomic number are located in the nucleus of the element. Elements in group 1 have one valence electron;
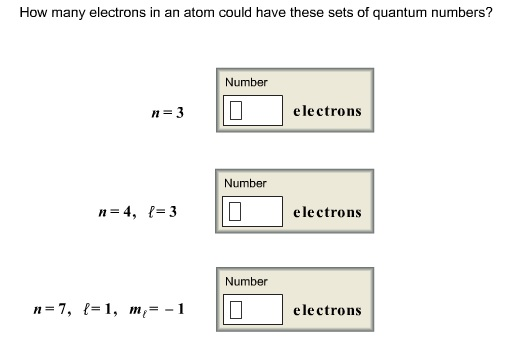
To find out the number of electrons in an element you must add the protons and neutrons and subtract that number with the atomic mass, this will determine the amount of. The number of electrons in a neutral atom is equal to the number of protons. We know that an equal number of protons of atomic number are located in the nucleus of the element and electrons equal to protons are in orbit outside the nucleus.